Niels Bohr's Atomic Model Theory
Denmark
Science
Technology
4 min read
Updated By: History Editorial Network (HEN)
Published:
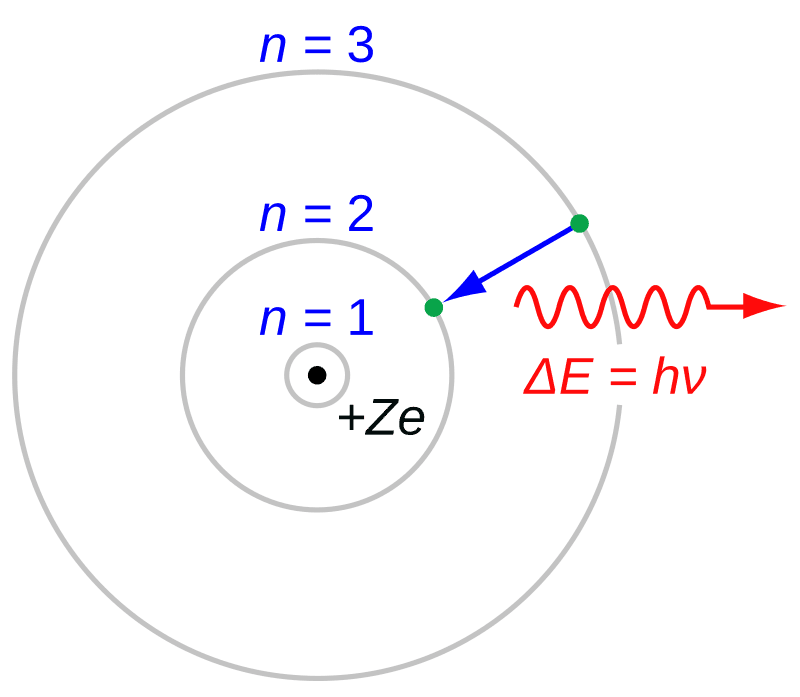
Danish physicist Niels Bohr introduced his model of the atom, revolutionizing the understanding of atomic structure. Born in Denmark, Bohr had been working on the mysteries of atomic behavior, specifically the arrangement of electrons within an atom. Building on Ernest Rutherford's nuclear model, Bohr proposed a new model where electrons orbited the nucleus in fixed energy levels or shells.
Bohr's model incorporated the newly emerging quantum theory, suggesting that electrons could only occupy certain energy levels. This breakthrough explained the stability of atoms and the emission of specific frequencies of light. Known as the Bohr Model, it was crucial in bridging classical physics with the developing quantum mechanics.
The impact of Bohr's model was profound, laying the foundation for further advancements in atomic theory and quantum mechanics. It provided a simple yet effective explanation of atomic behavior that was later expanded upon by scientists like Schrödinger and Heisenberg. Bohr's work led to the development of quantum theory as we know it today, shaping the world of physics and chemistry.
The concept of discrete energy levels and electron configurations proposed by Bohr revolutionized the field of atomic physics, enabling scientists to predict and understand chemical reactions, materials properties, and even biological processes at the atomic level. Bohr's model remains a fundamental concept in the teaching of chemistry and physics, highlighting the elegance and complexity of the atomic world.
Bohr's groundbreaking model of the atom has inspired generations of scientists and researchers, unlocking new realms of understanding in the microscopic world. His legacy endures in the continued exploration of quantum phenomena and the quest for a comprehensive theory of everything.
#NielsBohr #AtomicTheory #QuantumMechanics #BohrModel #ScientificBreakthrough
